


“Periodic Table ” by そらみみ is licensed under CC BY-SA 4.0
Batteries are available in many shapes and sizes, from the tiny silver disc powering a wristwatch to the battery used to start a semitrailer. Beyond size and shape, there are dozens of battery chemistries to choose from.
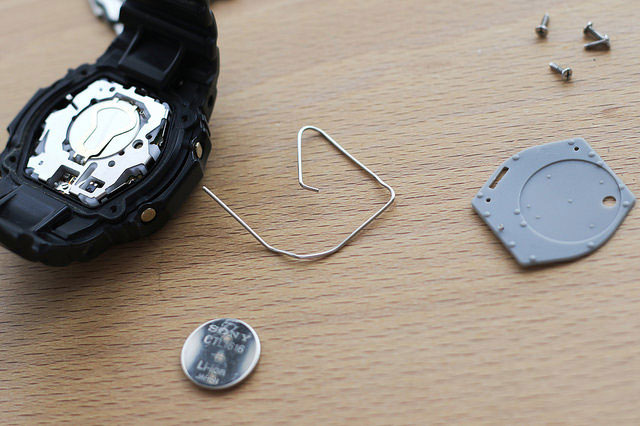
“Battery Replacement” by Miki Yoshihito is licensed under CC BY 2.0


![]() Lithium iron phosphate (LiFePO4)
Lithium iron phosphate (LiFePO4)
Batteries are not a one-size-fits-all solution. Selecting the best battery for an application requires knowing the load requirements and operating conditions.
Lithium batteries provide power at almost full capacity regardless of the discharge rate. When they are discharged at a high rate, no change in power output is seen until the last 10 or 20%. At this point power is still available, but at reduced voltage and rate.
Overall capacity (in amp hours) can be deceptive because a lithium battery can be deeply discharged, effectively using nearly all of the rated capacity. By contrast, lead-acid batteries are held to 50 to 70% of the capacity during discharge in order to extend battery life. For this reason, many people consider a lithium battery of the same capacity to actually have roughly twice the usable capacity of a lead-acid battery.
Lead-acid Best Uses
Deep-cycle Batteries: A deep-cycle battery is designed for maximum energy storage capacity ![]() and high cycle count, or long life, and is rated in amp hours (Ah). These attributes are achieved by installing fewer and thicker lead plates with limited surface area. Typical applications for deep-cycle batteries are boats, golf carts, wheelchairs, solar applications, RVs, and uninterruptible power supply (UPS) systems.
and high cycle count, or long life, and is rated in amp hours (Ah). These attributes are achieved by installing fewer and thicker lead plates with limited surface area. Typical applications for deep-cycle batteries are boats, golf carts, wheelchairs, solar applications, RVs, and uninterruptible power supply (UPS) systems.
Engine-starting Batteries: Starter batteries are made for maximum power output and are usually rated in cold-cranking amps (CCA). The battery manufacturer achieves this maximum, short-burst output by combining many thinner lead plates to obtain larger surface area for maximum conductivity. Typical applications for engine-starting batteries are cars and motorcycles.
Battery storage capacity and deep cycling are less important in automotive applications because the battery is being recharged while driving. If continuously cycled, the comparatively thin lead plates of a starter-type battery would wear down rather quickly.
LiFePO4 Best Uses
The energy density of a lithium battery is significantly higher than other battery chemistries. For example, a lithium battery that weighs 35 kg will provide the same amount of energy as a lead-acid battery that weighs 70 kg. This is a highly desirable feature when considering the portable power market. Additionally, lithium batteries can endure more charge/discharge cycles than other chemistries.
Early cell phones required large bulky batteries to operate. If you are old enough to remember the 1970s, then you may remember that a portable communications radio often required a battery that weighed more than the radio itself. With lithium batteries, cell phones and radios today are much lighter in weight with much more power.
The conversion efficiency of a battery denotes how efficiently it converts an electrical charge into chemical energy and then back again. A higher efficiency (expressed as a percentage) means that less energy is converted into heat (heat is lost energy) and that the battery can be discharged faster without overheating—assuming all other factors are equal.

Lead-acid Efficiency
Lead-acid batteries are not 100% efficient at converting charging amp hours back into stored amp hours. One of the main reasons why lead-acid batteries dominate the energy storage market is that the conversion efficiency of lead-acid cells is 85 to 95%. Generally speaking, this is often much higher than other types of rechargeable battery technologies. However, even at 90% efficiency, it will take approximately 110 Ah of charge to replace 100 Ah of consumed capacity from a lead-acid battery.
The lower the internal resistance of a battery, the better its conversion efficiency will be. This is another reason to avoid the buildup of “rock content”, which increases the resistance of the battery similar to the way a buildup of solutes within a pipe decreases the flow rate of water through the plumbing.
LiFePO4 Efficiency
The conversion efficiency of lithium batteries is virtually 100%. Lithium batteries have a very low internal resistance, and that is one reason they are both more efficient and more powerful than other batteries, pound for pound.
No other batteries are as close to 100% efficient at converting charging amp hours back into stored amp hours. The efficiency of lithium batteries and their low internal resistance are some of the reasons why they may recharge three to four times more quickly than lead-acid batteries. However, this also means they put a higher demand on conventional alternator or generator charging systems, so the entire system should be engineered with these differences in mind.

The voltage of a battery is the amount of electromotive force (or the amount of push) that moves electrons from negative to positive fields.
Lead-acid Voltage
The voltage of a lead-acid battery is a direct indication of its state of charge (SOC). Voltage is a function of the specific gravity ![]() of the electrolyte at the place in the battery where the chemical reaction occurs. Specific gravity is a measure of the health of the electrolyte in a lead-acid battery.
of the electrolyte at the place in the battery where the chemical reaction occurs. Specific gravity is a measure of the health of the electrolyte in a lead-acid battery.
LiFePO4 Voltage
A battery management system (BMS) ensures that individual battery cells are charged and maintained optimally. In a working configuration, lithium batteries usually require unique charging times, voltages, and amperages, and they can be easily and permanently damaged if they are not used with a proper BMS ![]() .
.
Cell damage can range from significantly shortened life to general poor performance, and in extreme cases a damaged cell can overheat, causing an explosion or fire. While it will not spew caustic electrolyte like a lead-acid battery, it will likely be a memorable event.

We consider ourselves authorities in portable power and students of its application. STIKopedia reflects that and is updated continuously as the industry grows and evolves.
Questions? Comments? Click the compass at the upper left to contact us.
Lead-acid batteries are the most commonly used batteries and come in several different configurations. The oldest of the lead-acid battery types are flooded-cell (or wet-cell) batteries and can be either the sealed or the open variety. In both types, the electrolyte evaporates due to charging, age, or ambient heat.
In the mid 1970s, a “maintenance-free” valve-regulated lead-acid (VRLA) battery was developed.
VRLA batteries can be absorbed glass mat (AGM) or gel cells. Solar Stik uses AGM batteries in its lead-acid products.




VRLA batteries remain under constant pressure of 1–4 psi. This pressure helps the recombination process during charging when more than 99% of the hydrogen and oxygen generated are turned back into water.
Unlike the flooded lead-acid battery, VRLA batteries are designed with a low overvoltage potential, which prohibits the battery from reaching its gas-generating potential during charge. This safeguard prevents excess charging, which would cause gassing and electrolyte depletion.
History of Lead-acid Batteries
Lead-acid is the oldest rechargeable battery technology in existence. Invented by the French physicist Gaston Planté in 1859, lead-acid was the first rechargeable battery to be used in commercial applications. More than one hundred fifty years later, we still have no real cost-effective alternatives for cars, boats, RVs, wheelchairs, scooters, golf carts, and UPS systems.
The lead-acid battery is still the most widely used 12 V energy storage device. A lead-acid battery is an electrical storage device that uses a chemical reaction to store and release energy. It uses a combination of lead plates and an electrolyte to convert electrical energy into potential chemical energy and back again.
There are many newer battery technologies available in the marketplace. However, lead-acid technologies are better understood and are widely accepted as the standard by which all other batteries are measured. Newer technologies often have operational constraints, including maximum and minimum operating temperatures and special charging requirements that make them less versatile and useful for the average consumer in everyday applications.
Solar Stik uses only lithium iron phosphate (LiFePO4) battery chemistry in its lithium-ion energy storage products because it has safety characteristics similar to lead-acid batteries. LiFePO4 uses a nonflammable electrolyte, so when it’s completely discharged it becomes inert, making it safe for users.
In some lithium-ion polymer batteries, improper charging and storing can cause the formation of crystalline “needles” that can puncture the internal separator, resulting in failure or fire. This is not the case with LiFePO4 batteries because the reactants that store the charge are not flammable. All other lithium battery chemistries are volatile, reactive, and flammable, and if they do overheat and catch fire, conventional halon fire extinguishers will not put out the fire.
Common LiFePO4 cell types include cylindrical and prismatic (LiFePO4 chemistry is not packaged in pouch cells, another lithium cell type). It is easy to see how these were named, as they are actual descriptions of their physical attributes; they look like what they sound like.


Cylindrical
LiFePO4 cylindrical cells are all made of the same basic components. Each cell, and the entire battery, is enclosed by a resilient plastic container. Inside the container there is a “rolled” foil, and between the foil there is a layer of permeable “separator” material. A safe, nonflammable electrolyte (unique to LiFePO4) is added to each cell and saturates the “foil” and “separator”. The battery terminals are typically threaded (rather than posts) so that heavier-duty connections can be made to the load.
 Prismatic
Prismatic
LiFePO4 prismatic cells make optimal use of space by using the layered approach. Small, flat versions are used in mobile devices where space is at a premium. The terminals can be oriented in any direction, which is an important feature for handheld devices. The smaller formats often have a softer, more flexible exterior and are sometimes referred to as pseudo-prismatic jellies.
Larger versions of the prismatic format are used to power vehicles and are often housed in welded aluminum housings. Stronger exterior housings are often required to compensate for the structurally softer inner construction of the prismatic format. Less efficient thermal management is inherent in their design, and overheating can reduce the life cycle and cause the cells to swell. If this occurs, remove and replace the battery before there is any damage to the component using the battery.
LiFePO4 is slightly less powerful than other commercially available lithium chemistries, but for many applications, the safety of its chemistry makes it the best choice despite its lower energy density. A LiFePO4 battery can be installed safely in any orientation. Safety vent valves are usually not required because the battery management system (BMS) will not allow the battery to overheat and vent gasses.
History of Lithium-ion Batteries
Experimental lithium batteries were developed as early as 1912, but it took nearly 70 years before a commercial lithium battery was developed for a wide market. Today, lithium batteries are most associated with enhancing “portable” capabilities. For example, they are the standard battery technology for high performance in portable electronics ranging from cell phones to laptop computers. There is a diverse family of lithium chemistries available. At first glance, they might all seem to be the same, but there are exploitable, distinct differences between them. The unique nature of the various chemistries allows each type to fill special application niches.
Even with wide market adoption in the early 1990s, as societal demands for lightweight portable electronics was burgeoning, the high cost barrier and complexities in battery management circuits would prevent lithium batteries from being used widely in support of larger devices or in scaled energy-storage systems such as large vehicles or uninterruptible power supply (UPS) systems.
Today, lithium battery technology continues to evolve at a rapid pace. Manufacturers, driven by demands from new applications, are constantly pushing the envelope by making changes in the chemistry and structure in search of improved battery life and greater energy density.

Battery Voltage
Battery voltage, or state of charge (SOC), of a lead-acid battery can be estimated by measuring the open (no load) battery terminal voltage using a digital voltmeter. Prior to measuring, the battery must have rested for 4 to 8 hours after charge or discharge and resided at a steady room temperature. With these conditions met, voltage measurements provide an amazingly accurate SOC for lead-acid batteries.

Specific Gravity
Specific gravity can be measured in wet-cell batteries with removable caps that provide access to the electrolyte. To measure specific gravity, you must use a tool called a temperature-compensating hydrometer, which can normally be purchased at an auto parts store or tool supply.
Load Testing
Load testing removes and measures the amps from a battery, similar to what happens when you start the engine of a car. Some battery companies label their battery with the amp load for testing. This number is usually about half of the CCA rating. A battery rated at 500 CCA would therefore be load-tested at 250 amps for 15 seconds.
A load test can only be performed if the battery is at or near a full charge. Some electronic load testers apply a 100-amp load for 10 seconds, and then display battery voltage. This number is then compared to a chart on the tester, which compares common load testing results to CCA ratings to determine battery condition.

Flexible solar PV panels fuse form factor with capability and deliver maximum power generation with minimum weight. Flexible panels use amorphous silicon or copper indium gallium selenide (CIGS) thin-film technology, which can be used with many substrate options that allow flexible panels to be folded or rolled.
Solar Stik uses extremely rugged, paper-thin, flexible PV panels that can withstand harsh conditions.
As the name implies, thin-film solar PV cells lack the thickness of other PV technologies. Composed of a very thin layer of substance on a substrate, today’s thin-film cells are one percent as thick as the first manufactured silicon solar cells.
Foldable or rollable thin-film panels make storage and transport convenient. For low-power applications that require portability, thin-film solar PV panels are an excellent option.

Numerous thin-film solar PV technologies exist today. However, they are slightly less efficient than other types of PV cells, so more surface area is required to generate the same amount of power. Most thin-film panels are designed for single-device applications, like recharging a battery-operated device.
The two most common types of thin-film solar PV panels are amorphous silicon and copper indium gallium selenide (CIGS).
![]()
Amorphous Silicon Solar PV Panel
Amorphous silicon is the oldest thin-film technology and arguably the best. When laid on a substrate, amorphous silicon does not require a grid configuration to conduct electricity, allowing it to be used on large areas with ease. However, it does not conduct as well as crystalline silicon solar PV cells used in rigid panel technology because the connections between the silicon atoms are not as consistent. This inconsistency results in interrupted electron flow.
Numerous substrate materials can be used with amorphous silicon, making the technology highly adaptable. Polymer plastic is one option for substrate. Because polymer plastic is flexible and able to be folded or rolled, it excels in applications requiring ease of storage or transport.
Amorphous silicon solar PV panels perform better in low light intensities. This makes amorphous silicon a good choice for environments with interrupted sunlight or dusty conditions.

Copper, Indium, Gallium, Selenide (CIGS)
Copper, indium, gallium, and selenide comprise the photoelectric layer of a CIGS solar cell. The principles behind the operation of CIGS PV cells are the same as those for silicon cells, like those used in mono- and polycrystalline solar PV panels.
With CIGS cells, copper acts to receive electrons in a fashion similar to the positive layer (P-type silicon) of a silicon cell. Selenium provides extra electrons to act in the same way as the negative silicon layer (N-type silicon). These materials can be placed onto a variety of substrates, including thin flexible steel, glass, and polymers. Flexible steel is the most widely used because of its resistance to the high temperatures needed to lay the PV elements on the backing sheet.
CIGS panels have a higher-rated output per square foot of surface area than amorphous silicon panels, which allows for relatively smaller CIGS panel sizes to achieve an equal amount of power. However, CIGS panels occasionally require direct exposure to sunlight—a process known as “light soaking”—before they can be used after being stored in dark, hot conditions.

Rigid solar PV panels are ideal for stationary applications that require maximum power and a small installation footprint. They are the first generation of solar PV panels, provide more power per square foot than other PV panel types, and are highly durable. Rigid panels do not degrade significantly over time, making them a good choice for long-term investment.
Solar Stik uses both multi- and monocrystalline, glass and non-glass—impact-resistant and shatterproof—rigid panels.

Rigid solar PV panels are typically made of glass or non-glass panels and aluminum frames. Rigid panels are among the best performing panels, but their physical characteristics make them a poor choice for certain applications—especially when portable power is desired.
Travel and storage can be difficult because rigid panels often contain breakable glass and cannot be folded.
The Solar Stik system design overcomes many of the physical challenges associated with the rigid panels. This results in portable power systems that draw from the best available PV technology.

The two main types of rigid solar PV panels are monocrystalline and multi- or polycrystalline.

A rigid monocrystalline solar PV panel is distinctly recognizable by the arrangement of the individual solar PV cells (squares with no corners) that appears as a uniform, flat color.
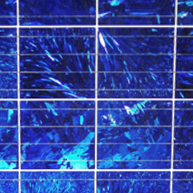
The surface of a rigid multi- or polycrystalline solar PV panel has the appearance of a rectangular grid and more of a bluish speckled color.
Performance differences between rigid solar PV panels can be experienced in high operating temperatures and shaded conditions. Monocrystalline panels perform better in higher external temperatures and full sun. Multi- or polycrystalline panels suffer performance losses in higher heats but have slightly higher outputs compared to monocrystalline panels when the panel is partially shaded.
The role of the battery management system (BMS) is simple: It controls the actual voltage of each cell, so that it doesn’t get too high or too low.
2Prolong the life of the battery
3Maintain the battery in a state in which it can fulfill the functional requirements of the application
To achieve these objectives, the BMS may incorporate one or more of the following functions:
Cell Protection Protecting the battery from out of tolerance operating conditions is fundamental to all BMS applications. In practice the BMS must provide full cell protection to cover almost any eventuality. Operating a battery outside of its specified design limits will inevitably lead to failure of the battery. Apart from the inconvenience, the cost of replacing the battery can be prohibitive. This is particularly true for high voltage and high power automotive batteries which must operate in hostile environments and which at the same time are subject to abuse by the user.
Charge Control This is an essential feature of BMS. More batteries are damaged by inappropriate charging than by any other cause.
Demand Management While not directly related to the operation of the battery itself, demand management refers to the application in which the battery is used. Its objective is to minimize the current drain on the battery by designing power saving techniques into the application’s circuitry and thus prolong the time between battery charges.
State of Charge (SOC) Determination Many applications require a knowledge of the state of charge of the battery or of the individual cells in the battery chain. This may simply be for providing the user with an indication of the capacity left in the battery, or it could be needed in a control circuit to ensure optimum control of the charging process.
State of Health (SOH) Determination The state of health is a measure of a battery’s capability to deliver its specified output. This is vital for assessing the readiness of emergency power equipment and is an indicator of whether maintenance actions are needed.
Cell Balancing The many cells that constitute a battery can sometimes charge and discharge at different rates, depending on the cell temperature, age, and factors that occur during manufacture of the cells. Over time, these differences will become amplified if they are not corrected. It is important that while charging and discharging, the balance between all cells is maintained. If cells are out of balance, the entire battery pack is shut off when the weakest cell drops below the lower voltage limit, even if other cells are still not completely discharged. Cell balancers are usually placed throughout the cell circuit in order to maintain a balanced charge throughout the entire battery and thereby extending the life of the battery.
To minimize performance differences, BMS components called Protection Circuit Modules or Protection Circuit Boards (PCMs or PCBs) are connected to each cell. The PCBs constantly monitor and report critical parameters of each cell and make small adjustments to correct for any differences between cells. Balancing or equalizing all parameters of each cell in a battery is critical to ensure full life and capacity from a lithium battery.
History (Logbook Function) Monitoring and storing the battery’s history is another possible function of the BMS. This is needed in order to estimate the SOH of the battery, but also to determine whether it has been subject to abuse. Parameters such as number of cycles, maximum and minimum voltages and temperatures, and maximum charging and discharging currents can be recorded for subsequent evaluation. This can be an important tool in assessing warranty claims.
Authentication and Identification The BMS also allows the possibility to record information about the cell, such as the manufacturer’s type designation and the cell chemistry, which can facilitate automatic testing, and the batch or serial number and the date of manufacture, which enables traceability in case of cell failures.
Communications Most BMSs incorporate some form of communications between the battery and the charger or test equipment. Some have links to other systems interfacing with the battery for monitoring its condition or its history. Communications interfaces are also needed to allow the user access to the battery for modifying the BMS control parameters or for diagnostics and testing.
Bricking a LiFePO4 Battery
As soon as the BMS senses that the cell voltage is too low to discharge, time is of the essence to place the batteries on charge. Failure to do this may cause a fatal error known as “bricking”. Once the batteries reach their internal disconnect voltage, the voltage can fall very rapidly in the internal cells, causing the battery to brick. This means that the battery cells are nonrecoverable, and the battery module must be replaced.
Specific gravity of the electrolyte can be defined as:
A measure of the density of the liquid electrolyte compared to the density of water at a specific temperature and pressure.
The chemical reaction takes place inside the pores of the active material on the battery’s lead plates. If the battery has just been charged, the electrolyte in the pores of these lead plates is very rich in sulfuric acid. As a result, the battery’s voltage will be high, perhaps as much as 13 to 14 volts. As the battery rests following a charge, its voltage slowly drops and then levels off as the electrolyte stabilizes its chemical state between the plates.

“Zero Gravity” by Scott Robinson is licensed under CC BY 2.0
A similar change in battery voltage occurs during discharge. During the battery discharge process, the electrolyte transfers its sulfur content to the lead plates. As the electrolyte loses sulfur, its specific gravity gets “lighter” or closer to that of water, indicating that the battery has been discharged. Because the specific gravity of the electrolyte is measurable, it can be used to determine the state of a battery’s charge and health. While a fully charged battery may read 12.68 volts, the voltage will drop and then stabilize at a somewhat lower value as a load is applied.
The change in voltage occurs even though the state of charge of the battery has not significantly changed. This is due to the local electrolyte in the pores of the plates becoming less rich in sulfur as the battery supplies current. As the battery discharges, electrolyte more like sulfuric acid enters the pores while electrolyte more like water exits the pores.
As discharge continues, the electrolyte in the pores eventually stabilizes at a specific gravity somewhat lower than the average value in the battery, producing the slightly lower battery voltage.
The operational characteristics of the lead-acid battery can be explained best by the terms capacity and cold-cranking amps (CCA).
Capacity is the amount of energy a battery can store. It is usually given in amp hours (Ah), or the amount of current measured in amps that the battery can provide over a period of one hour before rendering the battery discharged.
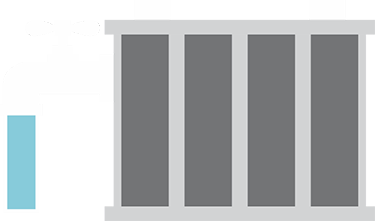
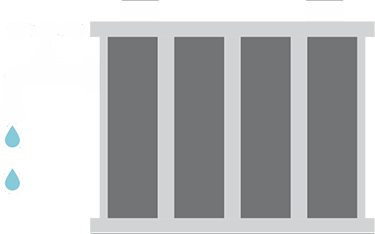
The secret of any battery’s runtime lies in the battery’s plate capacity. During charging and discharging, the lead on the plates gets gradually eaten away and the sediment falls to the bottom. The service life of a lead-acid battery can be measured by the thickness of the positive plates. The thicker the plates, the longer the life will be and the more energy storage you can expect.
The weight of a battery is another good indicator of the lead content and the life expectancy. Generally speaking, the heavier the battery, the more lead it contains and the longer it will last.
Most industrial flooded deep-cycle batteries use lead-antimony plates. Antimony is a metal that stiffens the lead plate and helps prevent battery failure due to structural failure of a plate. This improves the plate’s life but increases gassing and water loss. Antimony is not necessary in AGM batteries due to the rigid construction of the overall battery.
1953 automotive lead-acid battery
Cold-cranking amps (CCA) is the amount of energy a battery can deliver in short bursts. It is the maximum amount of current (amps) that a battery can deliver at 0 °F for 30 seconds without dropping below 7.2 volts. A high CCA battery rating is good, especially in cold weather. Starter batteries are often rated in CCA and are designed to deliver a short-duration burst of power, such as that required to start a vehicle.
Age and environmental conditions can affect the capacity and the CCA. As a battery ages, capacity and CCA will not degrade at the same rate. CCA tends to stay high through most of the battery’s life, but it drops quickly towards the end. If you drive a car, you’ve probably experienced this when, near the end of the battery’s life, suddenly the battery won’t start the car in the morning.
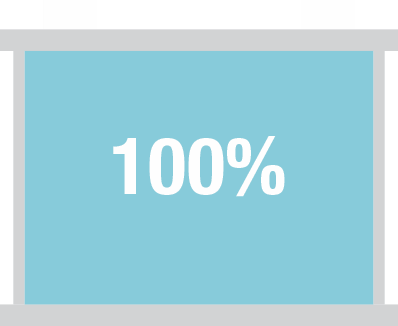

Capacity decreases gradually. A new battery is designed to deliver 100% of its rated capacity. As the battery ages, the capacity steadily drops and it should be replaced when its ability to store power falls below 70% of its original rating.
The overall health of a battery is most directly related to its capacity, not its CCA. As noted before, the CCA remains within the optimal range for most of a battery’s life, so performance and health declines will be most notable in the loss of capacity.
The illustration shows two fully charged lead-acid batteries, one with a high capacity and one that has aged. The buildup of visible “rock content” (crystalline formation, also called sulfation or memory) due to aging robs the battery of usable capacity, although the battery may still provide good cranking power.
Appliance efficiency is also known as load efficiency. As appliances consume less power, power source requirements also change. When designing a portable power system, purchasing highly efficient components can provide many benefits.
Appliance loads can often be matched to the electrical characteristics of the circuit. This will increase the system’s overall efficiency by allowing direct connection to the circuit without the need for additional power management devices to aid in the function.
The fewer management components used in a system, the more efficiently it will operate. For example, components such as inverters, converters, or similar devices used in a circuit to “adapt” appliances for use in a particular electrical circuit themselves require power to operate, and thus the total power required to operate the appliance is increased.
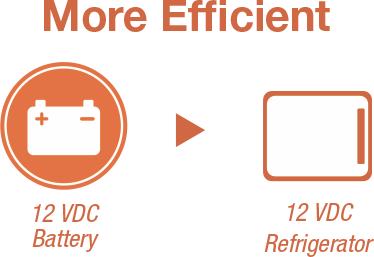


For example, a 12-volt direct current (DC) electrical circuit powered by a 12 V battery can directly support a refrigerator that also operates at 12 VDC. This setup will transfer power through the circuit more efficiently than if the refrigerator requires 120 V alternating current (AC) power. In the latter example, an inverter would be required.
It is prudent to shop around when looking for appliances because power consumption varies among models even within a particular appliance class. Purchasing an energy-efficient device can be more expensive up front, but could mean future savings in energy costs as well as a flexibility of use that makes the device compatible with a variety of portable power sources. When purchasing an electrical appliance, remember to ask if a 12 VDC adapter is available for the product.
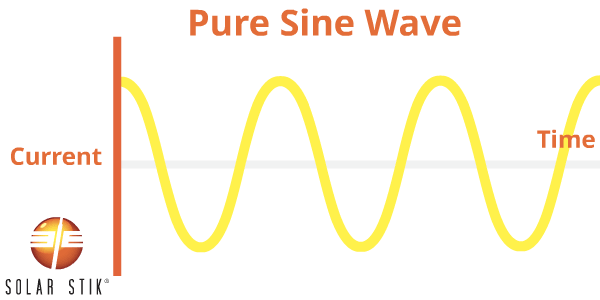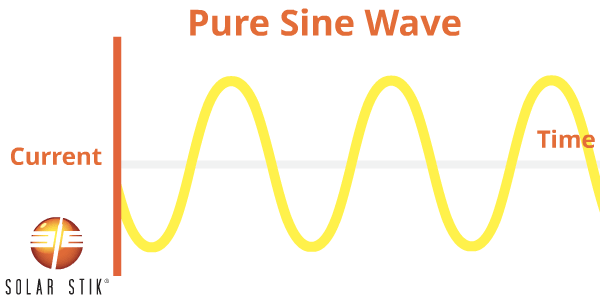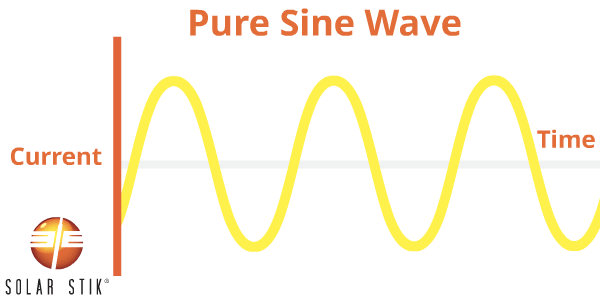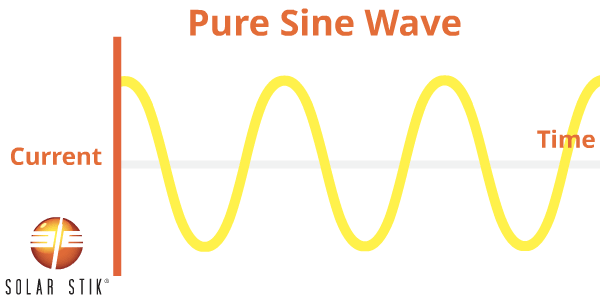
A pure sine (also referred to as a sinusoidal) wave can be produced by rotating machinery (a generator). This is the type of waveform provided by electric utility companies. This type of power is available anywhere an outlet is tied to the power grid, such as in homes or businesses.
A PSW inverter reproduces this waveform through the use of advanced internal circuitry.
A modified sine wave (also referred to as non-sinusoidal or step-wave) inverter is different from a pure sine wave power inverter because the modified waveform output is step-shaped.
AC appliances that are not specifically designed to work with this type of inverter waveform output may take more power to operate, thereby reducing the efficiency of the entire electrical system. For example, some appliance motors may produce more heat and burn out when they are operating.
Other appliances that use electronic controls will not be able to vary speed or temperature when using modified sine wave power. Some fluorescent lighting may not get as bright or may make buzzing noises. Appliances with digital clocks or electronic timers may not work properly with this type of inverter because the waves are rougher and cause extra noise to be created in the circuitry.
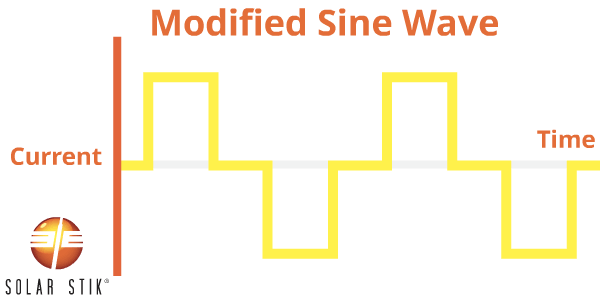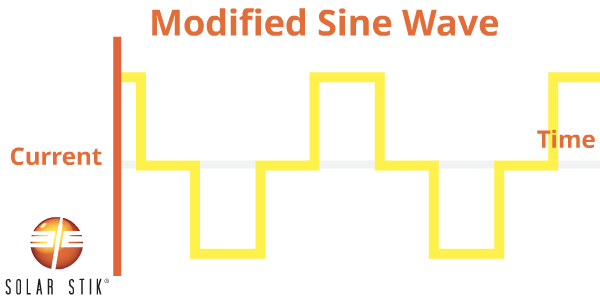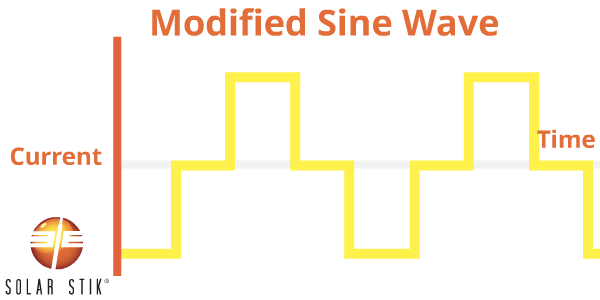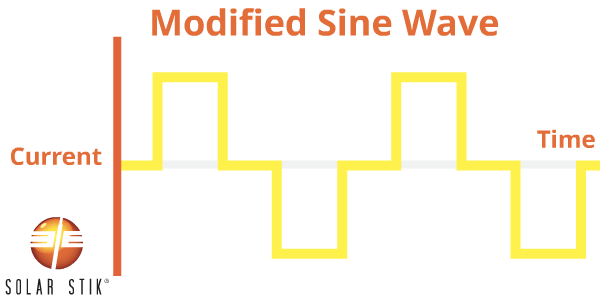
The following appliances may experience problems when operated from MSW inverters:

Low-wattage Inverters
Most vehicle starting batteries will support a low-wattage inverter for short time periods. Actual operating time will vary depending on the age and condition of the battery, the Ah capacity of the battery, and the AC appliance powered by the inverter. If you use a low-wattage inverter that is powered through a DC accessory socket, and the vehicle engine is turned off, you should periodically run the engine to recharge the battery.

Medium- and High-wattage Inverters
It is strongly recommended that only deep-cycle batteries be used for any inverter with a continuous output of 200 W or more. This will ensure that you have several hundred complete charge and discharge cycles. If you use a normal vehicle starting battery to support a medium- or high-wattage inverter, it will quickly fail after repeated charge/discharge cycles (since starting batteries are not designed to perform this type of work).
When the inverter operates power-hungry appliances with continuous loads for extended periods, it will drain the battery to the point where the battery has insufficient energy to support the inverter. In these cases, it’s a good idea to have additional deep-cycle batteries available to extend the appliance operating time.
Lead-acid batteries are the most commonly used batteries and come in several different configurations. The oldest of the lead-acid battery types are flooded-cell (or wet-cell) batteries and can be either the sealed or the open variety. In both types, the electrolyte evaporates due to charging, age, or ambient heat.
In the mid 1970s, a “maintenance-free” valve-regulated lead-acid (VRLA) battery was developed.
VRLA batteries can be absorbed glass mat (AGM) or gel cells. Solar Stik uses AGM batteries in its lead-acid products.




VRLA batteries remain under constant pressure of 1–4 psi. This pressure helps the recombination process during charging when more than 99% of the hydrogen and oxygen generated are turned back into water.
Unlike the flooded lead-acid battery, VRLA batteries are designed with a low overvoltage potential, which prohibits the battery from reaching its gas-generating potential during charge. This safeguard prevents excess charging, which would cause gassing and electrolyte depletion.
History of Lead-acid Batteries
Lead-acid is the oldest rechargeable battery technology in existence. Invented by the French physicist Gaston Planté in 1859, lead-acid was the first rechargeable battery to be used in commercial applications. More than one hundred fifty years later, we still have no real cost-effective alternatives for cars, boats, RVs, wheelchairs, scooters, golf carts, and UPS systems.
The lead-acid battery is still the most widely used 12 V energy storage device. A lead-acid battery is an electrical storage device that uses a chemical reaction to store and release energy. It uses a combination of lead plates and an electrolyte to convert electrical energy into potential chemical energy and back again.
There are many newer battery technologies available in the marketplace. However, lead-acid technologies are better understood and are widely accepted as the standard by which all other batteries are measured. Newer technologies often have operational constraints, including maximum and minimum operating temperatures and special charging requirements that make them less versatile and useful for the average consumer in everyday applications.
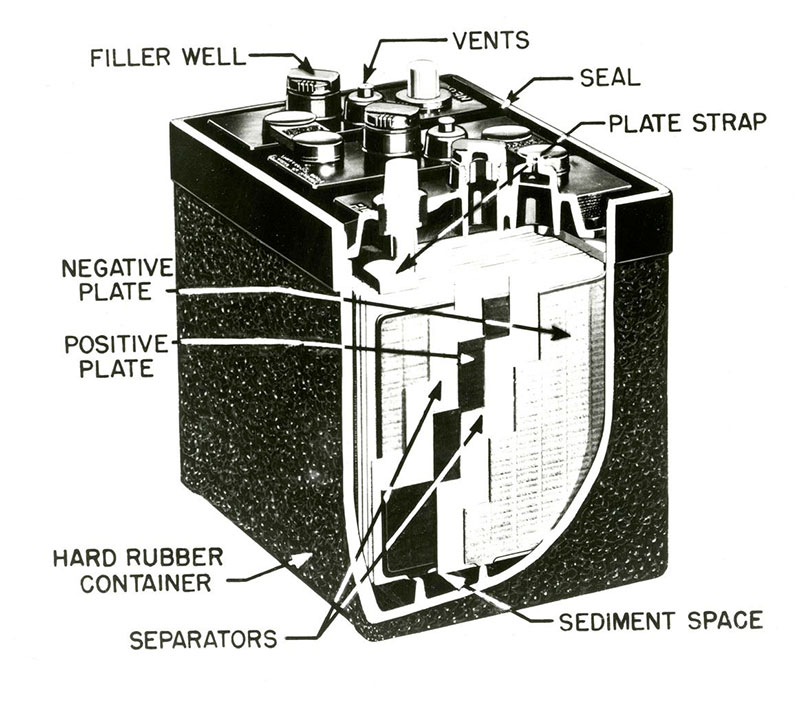
Lead-acid batteries are commonly made of five basic components:
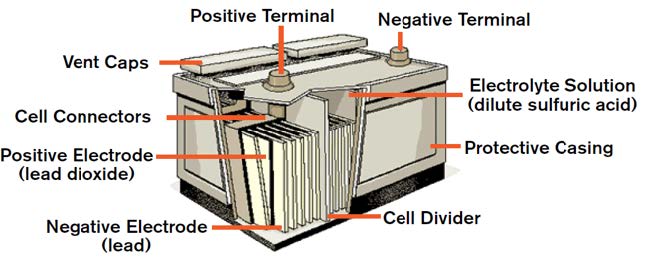
A battery cell is a container in which electrolyte and lead plates can interact. The electrolyte is usually a solution made up of 35% sulfuric acid and 65% water. The lead plates are treated with lead oxide and powdered sulfates to give them their positive and negative properties.
When the positive and negative lead plates are submerged in the battery’s electrolyte, a chemical reaction occurs. This reaction causes electrons to flow between the lead plates. The negative lead plate builds up an excess of electrons in a process called oxidation. This causes an electrical difference between the negative plate and positive plate.
The extra electrons on the negative lead plate want to displace the electrons on the positive plate in a process called reduction. However, the electrolyte solution of sulfuric acid and water ensures the electrons cannot travel directly to the positive plate. When the circuit is closed (with the help of a “conductive path”, or load, between the negative and positive plates), the electrons are able to travel to the positive plate. This, in turn, provides power to any appliance placed along the path.
This electrochemical process can be summarized as a reversible transfer of sulfate between the water and the lead plates during charging and discharging. As the battery is discharged, sulfate in the solution combines chemically with the lead plates of the battery to form lead sulfate. As the plates accumulate this sulfate, the electrolyte solution becomes more like water and less like sulfuric acid. The reverse occurs as the battery is charged. As charging current flows into the battery, the battery plates revert back to their original condition and the electrolyte reverts back to its original sulfuric acid content.
Lithium-ion batteries are made of the following basic components:
The exact chemistry is often patented and proprietary to each battery maker.
When a charge is applied to a lithium-ion battery, electrons flow between the internal components. The basis of this reaction is the lithium metal binding and unbinding with the other chemicals in the electrodes at the ionic level. As power is drawn out of the battery, the metal moves from one electrode to the other, and when the battery is charged, it moves back to the original state. The metallic lithium ions literally move through the separator material.